redox titration lab report
Redox indicators the indicator has different color at reduction and oxidation state. In this experiment you will use potassium permanganate KMnO4 as the titrant in the.

Redox Titration Pdf Titration Chemistry
Experiment 7 Redox Titration of Vitamin C Introduction In this experiment you will be acting as the quality control laboratory for a pharmaceutical manufacturer.

. The sample contains sodium oxalate and an inactive substance impurity. A redox reaction helps determine the concentration of a solution containing oxidizing or reducing agents. Introduction Oxidation-reduction reactions also known as redox reactions are reactions that usually involve transfer of electrons.
Now its time for the titration. Repeat step 12 until the solution turns light pink. Each Erlenmeyer flask had 0 g of sodium oxalate transferred into them with water and sulfuric acid later added.
Starch changes to deep blue color when excess amount I. Redox titration a measured sample of the unknown is titrated against a standard solution of a. In this experiment permanganate will be reduced by oxalate C 2 O 4 2-in acidic conditions.
The purpose of this lab was to standardize potassium permanganate solution and to determine the percent by mass of iron in a salt. Determination of Fe by Redox Titration Matt Cuff Quant 320L October 21 2011 Abstract In this experiment the percent of iron in an unknown sample will be determined by using a redox titration and then compared to a different method. Redox Titration Lab Report.
Slowly add one drop of potassium permanganate to the flask at a time. The concentrations of redox-active species can be determined by redox titrations. Redox titration is based on an oxidation-reduction reaction between the titrant and the analyte.
Oxidation reduction reactions involve transfer of electrons. Redox Titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the titrant and the analyte. To determine the number of electrons transferred oxidation states are assigned.
Introduction Oxidation-reduction reactions also known as redox reactions are reactions that usually involve transfer of electrons. Oxalate reacts very slowly at room temperature so the solutions are titrated hot to make the procedure practical. 20008 g KIO3 x 1 mol214 g KIO3 00093495327 mol KIO3 00093495327 mol KIO3 0500 L 00186990654 M KIO3 6 Na2S2O3 KIO3 6 H I- 3 H2O 3 S4O62- K.
Vitamin C is important because it functions as a cofactor in the synthesis of collagen protein metabolism iron absorption and the healing of wounds. Ascorbic acid C6H8O6 more commonly known as vitamin C is a vital vitamin in mammals. The unbalance redox reaction is shown below.
DETERMINATION OF ASCORBIC ACID BY REDOX TITRATION Name. Carmen Ontiveros Arlette Renteria. After we added one gram of the unknown substance and recorded the new mass of the flask.
Unknown s olid sample dissolved in water. In fact the recommended daily intake of Vitamin C is 75-90 mg per day. Between both known and unknown solutions such as acidbase reaction or redox.
CHEM 1A Lab Department of Chemistry California State University Fresno CA 93740. Titration method requires two solutions one of which is of known concentration. However in this lab experiment you will perform titrations for an oxidation-reduction reaction often called redox reaction and will find that the stoichiometry is not 11 and that the reaction is self-indicating.
A primary standard which in this case is ferrous ammonium sulfate will be used to standardize. The product line that you support produces 100-mg Vitamin C supplements. Titration is based on some reaction.
STANDARDIZATION OF AQUEOUS SODIUM THIOSULFATE SOLUTION 1. Cry H2O This equation can be used to convert moles of the dichloride ion to moles of the unknown iron to determine the percent of iron contained in the sample To prepare the acid mixture add 125 ml of both concentrated phosphoric acid ND sulfuric acid to 500 ml of denizens water and mix well. Redox Titration Lab Report.
A redox reaction is a chemical reaction that only happens when oxidation and reduction process take place simultaneously. Redox titration includes oxidation half reactions and reduction half reactions. Oxidation states of atoms.
Substance that will oxidize or reduce the unknown. And the other of unknown concentration. Non redox indicator change color when excess amount of titrant exists eg.
The permanganate oxidation of Fe 2 to. In the present experiment you will take a. Redox titration is the type of titration based on redox reaction between the analyte and titrant.
In this experiment one of the main types of chemical reactions oxidationreduction or redox will be used in the titration analysis of an iron compound. The purpose of this experiment was to determine how much KMnO4 was needed to titrate approximately 1 mL of an Unknow X101 concentrated solution of Oxalic Acid. Lab Manual Although vitamin C is.
The concentration of potassium permanganate solution KMnO4 was not given in the experiment that was carried out. Latifzadeh 28 June 2022 Iron Analysis by Redox Titration Lab Report Procedure In this lab experiment we first calculated the mass of an empty 250 mL Erlenmeyer mask and recorded its mass. These types of titrations sometimes require the use of a potentiometer or a redox indicator.
Phosphoric acid is added to the titration mixture to form a colorless complex with. Sample containing iron add acid to dissolve it thereby converting all the iron to. In this lab we began with the standardization of KMnO 4 with sodium oxalate.
Performing this particular lab also aided with the understanding of. Potassium permanganate KMnO 4 is a common chemical found in most laboratories. CHM 3120L ANALYTICAL CHEMISTRY I LABORATORY REPORT EXPERIMENT.
Fe 3 is carried out in an acidic solution to prevent the air oxidation of Fe 2. You are to determine the average amount of Vitamin C per tablet in a sample of tablets and. Redox Titration Goal To determine the mass of iron in a supplement pill using redox titration.
General Chemistry Professor L. Swirl after adding each drop. REDOX TITRATION Learning Outcomes Upon completion of this lab we will be able to apply the principles of titration that were previously discussed and perform to an oxidation-reduction reaction.
MnO 4-2 C 2 O 4- Mn2 CO 2. The normally yellow or orange Fe 3 ion which markedly sharpens the appearance of. In addition we will evaluate the percentage of hypochlorite in bleach.
That is there is no indicator needed. To determine the number of electrons transferred oxidation states are assigned. Redox Titration Goal To determine the mass of iron in supplement pill using redox titration.
Gancenia 1 Gancenia Andrea Monique CHE 110-03. The reduction of permanganate requires strong acidic conditions. Experiment 6 REDOX Titration Tate Morrison INTRODUCTION The purpose of this lab was to standardize a potassium permanganate KMnO4aq solution and then use that standardization to determine the amount of sodium oxalate Na2C2O4s present in a Thorn Smith Chemists sample.

Lab Iron Determination By Redox Titration

Redox Lab Report Docx Redox Titration And Determination Of Fe Course Chemistry 1300 Da1 Date Of Experiment Thursday June 15 2017 Abstract The Course Hero
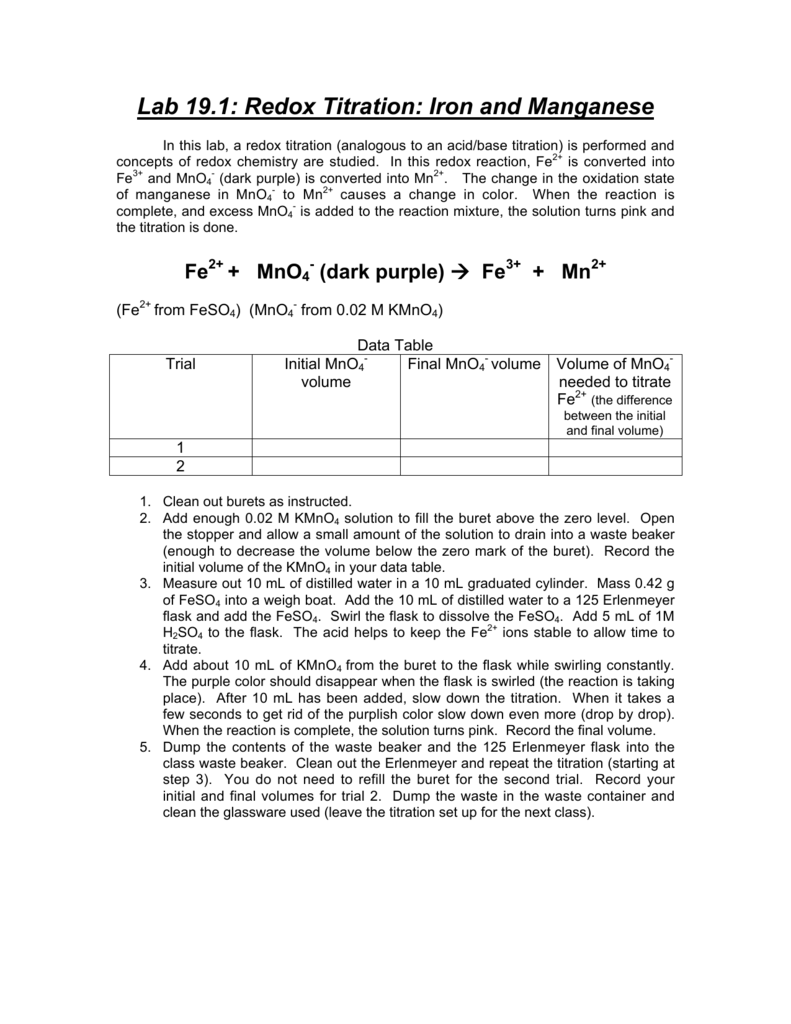
Lab 19 1 Redox Titration Iron And Manganese

Determination Of Iron By Redox Titration

Report 3 Warning Tt Undefined Function 3 Lab Report For Experiment 3 Visual Redox Studocu

Solved Experiment 8 Determination Of The Amount Of Sodium Chegg Com

5 Redox Titration Lab Report Docx Experiment Redox Titration And Determination Of Percent Iron Fe Daniela Garcia Meghan Hass And Lucero Course Hero

Determination Of Vitamin C By Redox Titration With Iodate Pdf Titration Chemistry

Formal Lab 2 H2o2 Redox Titration

Lab 2 Iron Via Redox Titration Mary Athos 13275122 Lab Report 2 Proficiency Testing Analysing Studocu

Acid Base Titration Lab Report Great College Essay

Titration Redox Handwritten Notes Chemistry Notes Organic Chemistry Notes Handwritten Notes

Pdf Oxidation Reduction Titrations I Determination Of Oxalate
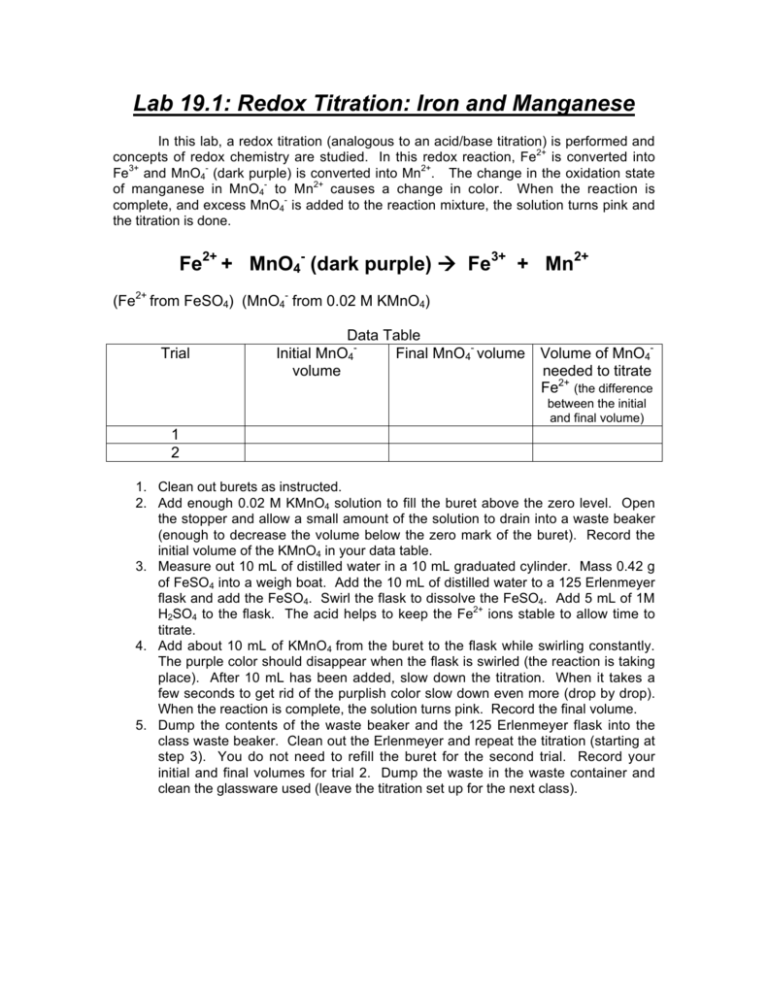
Lab 19 1 Redox Titration Iron And Manganese

Redox Titration Lab Report Redox Titration Abstract The Purpose Of This Lab Was To Standardize Studocu

Formal Lab 2 H2o2 Redox Titration

Determination Of Iron Ii By Redox Titration Lab Purpose
